| Ch 10. Rankine Cycle | Multimedia Engineering Thermodynamics | ||||||
|
Rankine Cycle |
Reheat | Regeneration | Cogeneration | ||||
| Ideal Rankine Cycle | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| Search |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| Author(s): |
| Meirong Huang |
| Kurt Gramoll |
| ©Kurt Gramoll |
| |
||
Thermodynamic cycles can be divided into two general categories: power cycles, which produce a net power output, and refrigeration and heat pump cycles, which consume a net power input. The thermodynamic power cycles can be categorized as gas cycles and vapor cycles. In gas cycles, the working fluid remains in the gas phase throughout the entire cycle. In vapor cycles, the working fluid exits as vapor phase during one part of the cycle and as liquid phase during another part of the cycle. Steam power plants run vapor power cycles with water as the working fluid. This section introduces the ideal cycle for vapor power cycle - Rankine cycle. |
||
| Rankine cycle - the Ideal Cycle for Vapor
Power Cycle |
||
|
|
Vapor power plants generate electrical power by using fuels like coal, oil or natural gas. The schematic of a vapor power plant is shown on the left. The entire power plant can be broken down into four major subsystems.
The focus of this chapter is subsystem A. The thermodynamic cycle in
subsystem A is called the Rankine cycle. Subsystem A consists of a boiler,
turbine, condenser and a pump. Fuel, burned in the boiler,
heats
the water to
generate superheated steam (subsystem B). This steam is used to run
the turbine which
powers
the
generator. Electrical energy is generated when the generator windings
rotate in a strong magnetic field (subsystem C). After the steam leaves
the turbine, it is cooled to its liquid state in the condenser by transferring
heat to the cooling water system (subsystem D). The liquid
is pressurized
by the pump prior to going back to the boiler. |
|
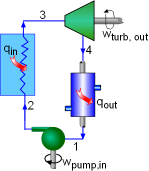 Schematic of the Rankine Cycle |
All four components associated with the ideal Rankine cycle are steady-flow
devices, and thus all four processes that make up the Rankine cycle can
be analyzed as steady-flow process. The kinetic and potential energy
changes of water are small relative to the heat and work terms, are
thus neglected. Energy analysis of the four components are given below. Pump (process 1-2): Pump pressurized the liquid water from the condenser prior to going back to the boiler. Assuming no heat transfer with the surroundings, the energy balance in the pump is wpump, in = h2 -
h1 |
|
Boiler (process 2-3): Liquid water enters the boiler and is heated to superheated state in the boiler. The energy balance in the boiler is qin = h3 - h2 Turbine (process 3-4): Steam from the boiler, which has an elevated temperature and pressure, expands through the turbine to produce work and then is discharged to the condenser with relatively low pressure. Neglecting heat transfer with the surroundings, the energy balance in the turbine is wturbine, out = h3 - h4 Condenser (process 4-1): Steam from the turbine is condensed to liquid water in the condenser. The energy balance in the condenser is qout = h4 - h1 For the whole cycle, the energy balance can be obtained by summarizing the four energy equations above. It yields, (qin- qout) - (wturbine, out - wpump, in) = 0 The thermal efficiency of the Rankine cycle is determined from ηth = wnet ,out/qin = 1 - qout/qin where the net work output from the cycle is wnet ,out = wturbine, out -
wpump, in |
||
 T-s Diagram of an Ideal Rankine Cycle |
The Rankine cycle is an ideal cycle if water passes through the four components without irreversibilities and pressure drops. The ideal Rankine cycle consists of the following four processes, as shown on the T-s diagram on the left:
|
|
| Actual Vapor Power Cycle
|
||
 Deviation of Actual Vapor Cycle from the Ideal Rankine Cycle |
The actual vapor power cycle differs from the ideal Rankine cycle as a result of irreversiblities in various components. The two common source of irreversiblities are the friction and undesired heat loss to the surroundings. Fluid friction causes pressure drops in the boiler, the condenser, and the connecting pipes. To compensate for these pressure drops, the water needs to be pumped to a higher pressure. Heat loss from steam to surroundings takes place when steam flows through the connecting pipes and the various components. To maintain the same work output, more heat needs to be transferred to the steam in the boiler. The deviation of actual pumps and turbines from the isentropic ones can be accounted for by utilizing adiabatic efficiencies: where the subscript a means the actual value and subscript s means the isentropic value. |
|

