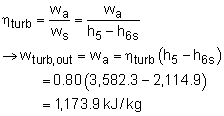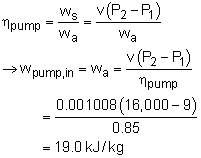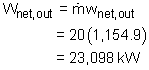| Ch 10. Rankine Cycle | Multimedia Engineering Thermodynamics | ||||||
|
Rankine Cycle |
Reheat | Regeneration | Cogeneration | ||||
| Ideal Rankine Cycle | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||
|
|
A new system has just started operating in Neon's power plant. An analysis needs to be done to make sure that the cycle works well. The thermal efficiency and the net work output need to be determined. Assumptions:
|
|
|
Saturated Water Temperature Table |
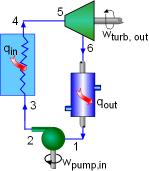
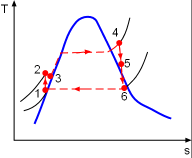
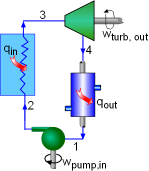
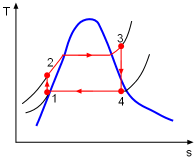
Model the cycle in the new section of the power plant as a Rankine cycle. The schematic and the T-s Diagram of this cycle is shown on the left. (1) Determine the work output of the new system The net work output of the cycle equals the difference between the turbine work output and the pump work input. The enthalpy at each state needs to be determined first, which can be obtained from the water table. State 1: Compressed liquid water State 3: Compressed liquid water State 4: Superheated vapor State 5: Superheated vapor State 6: Saturated mixture where s6s and h6s are the entropy and enthalpy at state 6 if process 5-6 is isentropic. Since the isentropic efficiencies of the pump and turbine are given, the
turbine work and the pump work can be determined as following: Therefore, the net work output per mass is The power produced by the new system is determined from (2) Determine the thermal efficiency Heat input to the cycle from the boiler is determined from The thermal efficiency of the cycle is |
|
|
|
(3) Determine the thermal efficiency of the ideal Rankine cycle The properties at each state of the ideal Rankine cycle can be obtained from the water table. State 1: Saturated water State 2: Compressed liquid water State 3: Superheated vapor State 4: Saturated mixture Pump work input: Turbine work output: Hence, the net work output of the ideal cycle is Heat input to the ideal cycle can be determined from The thermal efficiency of the cycle is then determined as Compared with the actual Rankine cycle, the ideal cycle has a higher thermal
efficiency. |
|