| Ch 1. Basics | Multimedia Engineering Fluids | ||||||
|
Mass Density |
Ideal Gas Law |
Viscosity |
Surface Tension |
Vapor Pressure |
|||
| Vapor Pressure | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Fluid Statics |
| 3. Kinematics |
| 4. Laws (Integral) |
| 5. Laws (Diff.) |
| 6. Modeling/Similitude |
| 7. Inviscid |
| 8. Viscous |
| 9. External Flow |
| 10. Open-Channel |
| Appendix |
| Basic Math |
| Units |
| Basic Fluid Eqs |
| Water/Air Tables |
| Sections |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The vaporization and condensation processes will be introduced in this
section. The concept of vapor pressure is then presented. The
conditions when boiling and cavitation occur will be discussed as well. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vaporization and Condensation |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
When the molecules at the surface of a liquid or solid gain enough energy to overcome the cohesive force, the molecules will escape into the air. The substance undergoes a phase change and turns into vapor. This process is referred to as vaporization (e.g., evaporation and sublimation, see below). In general, the rate of vaporization increases with the temperature. The process of phase change from liquid to vapor is called evaporation.
For example, water will evaporate into vapor when the temperature reaches
100oC (for atmospheric pressure at sea level). A phase change
directly from solid to vapor is called sublimation. An example of a sublimation
process is dry ice at room temperature. The dry ice
will become vapor. Also, ever
wonder
why moth balls will "disappear" over a period of time, but one
can still smell the odor? This is another example of sublimation. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor Pressure |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
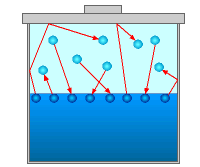
Now consider a closed container partially filled with a liquid, as shown in the figure. As the liquid molecules at the surface gain sufficient energy to escape into the air (evaporation), some of the liquid molecules will collide with the wall or air molecules, bounce back and re-enter the liquid (condensation). Over a period of time, the system will reach a steady-state where the rate of evaporation is the same as the rate of condensation. At this instance, the pressure exerted on the liquid surface by the liquid vapor is called vapor pressure. Vapor pressure is a fluid property, and it is a function of the temperature. Generally, the vapor pressure increases with temperature. The value of vapor pressure for water and is summarized in the table below as a function of temperature. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling and Cavitation |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Boiling |
Boiling will occur when the absolute pressure of a liquid is less than or equal to its vapor pressure. One characteristic of the boiling process is the formation of vapor bubbles in the liquid. The formation and collapse of bubbles, primarily due to a reduction in pressure, in fluid flow is called cavitation (flow induced boiling). In engineering applications (e.g., pumps, turbines and hydraulic systems), it is a good practice to avoid cavitation because it can cause structural damage, produce noise, and reduce the overall efficiency of the system. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
at Various Elevations |
It is well known to backpackers that boiling of water is dependent on the current elevation above sea level. An increase in elevation reduces the atmospheric pressure. With a lower pressure, water (or any liquid) will boil at a lower temperature that matches the vapor pressure. For example, at an elevation of 4 km, the atmospheric pressure is about 60 kPa (see table at left). At that pressure, water will boil at about 86 C (see table above). This does not mean food will cook faster or slower, but boiling will just occur at a lower temperature. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Practice Homework and Test problems now available in the 'Eng Fluids' mobile app
Includes over 250 free problems with complete detailed solutions.
Available at the Google Play Store and Apple App Store.