| Ch 2. Pure Substances | Multimedia Engineering Thermodynamics | ||||||
| Phase |
Property Diagrams |
Property Tables |
Ideal Gas |
||||
| Phase and Phase Change | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
| |
||
| Introduction |
||
|
|
A student is working on his thermodynamics experiment about phase change. He boils room temperature water using a pan placed on the top of a electric heater at room pressure. A thermometer is used to measure the temperature of the substance in the pan. Known:
|
|
| Questions |
||
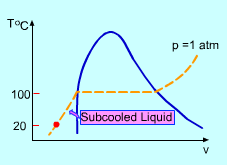 Phase Change Process on T-v Diagram |
The student needs to record the temperature
over a period of time. The records should include all phases
such as subcooled liquid, saturated liquid, saturated mixture, saturated
vapor, and superheated vapor. Please determine the time intervals between
each two records. |
|
| Approach |
||
|
||