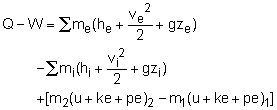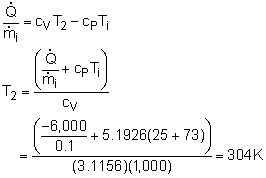| Ch 4. Energy Analysis | Multimedia Engineering Thermodynamics | ||||||
|
Steady-flow Process |
Steady-flow Devices (1) |
Steady-flow Devices (2) |
Steady-flow Devices (3) |
Unsteady-flow Process |
|||
| Unsteady-flow Process | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||
|
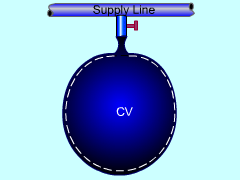
Balloons need to be filled for a welcoming party. Determine the heat that has to be removed to prevent the balloon from being overheated. Also determine the time needed to fill a single balloon. Assumptions:
|
||
|
|
Take the balloon as a control volume. The filling process belongs to unsteady-flow processes since the balloon is empty initially and contains some helium when the filling process ends. It has only one inlet and has no exit. The pressure in the balloon will reach the pressure in the supply line at the end of the filling process. If the temperature in the balloon at the end of the filling process is less than 50 oC, Minnie can use this supply line to fill her balloons. Hence, temperature in the balloon at the end of the filling process (T2) needs to be determined. (1) Determine the temperature in the balloon when the filling process ends. At the beginning of the filling process, the balloon is empty. If 1 denotes the initial state and 2 denotes the final filled state, then the mass at state 1 is m1 = 0 m2 - m1 = mi The energy balance for unsteady-flow process, can be simplified using the initial assumptions (v = 0, z = 0, ke = 0, pe = 0) and the mass terms, giving Q = - mihi + m2u2 It should be noted that work and potential energy are not really zero, but they offset each other. The work done to filling the balloon is assumed to be 100% converted into potential energy. Next, the enthalpy and the internal energy of Helium can be expressed as hi = cPTi Also, the total heat removed from the balloon by the cooling system during the filling process can be expressed as Substittuting hi, u2 and Q (recall, mi = m2) gives It is safe to fill balloons using this supply line. (2 ) Determine the time needed to fill a single balloon. Helium is an ideal gas and thus obeys the ideal-gas equation of state. P2V2 = m2 RT2 where Since the relation between the pressure and the volume of the balloon is given as P = 10 V, the volume of the balloon at state 2 can be determined. That is P2 = 10V2 Hence, the mass in the balloon at the end of the filling process is m2 = 120(1,000)(12)/(2.0769(1,000)(31+273)) The assumption states that the flow is steady from the supply line. Hence, It will take 3.8 minutes to fill one balloon. |
|