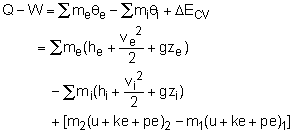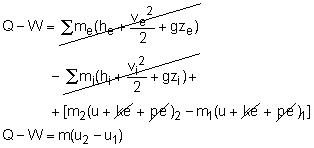| Ch 4. Energy Analysis | Multimedia Engineering Thermodynamics | ||||||
|
Steady-flow Process |
Steady-flow Devices (1) |
Steady-flow Devices (2) |
Steady-flow Devices (3) |
Unsteady-flow Process |
|||
| Unsteady-flow Process | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| Search |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| Author(s): |
| Meirong Huang |
| Kurt Gramoll |
| ©Kurt Gramoll |
| |
||
| Mass and Energy Balance of Unsteady-flow
Processes |
||
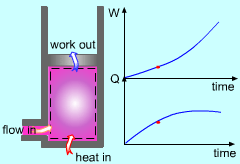 Unsteady-flow Processes |
In the previous sections, it was noted that nozzles, diffusers, turbines, compressors and other devices undergo a steady-flow process because of their long-time running consideration. But their startup and shutdown periods undergo transient operations since their states change with time. The flow processes involved are called unsteady-flow processes, or transient-flow processes. Unlike steady-flow processes, unsteady-flow processes start and end over some finite time period (Δt). An additional example of an unsteady-flow process is filling or discharging a tank. |
|
During an unsteady-flow process, the mass in the control volume changes with time. The mass balance for a system undergoing any process, can be used for control volume as where |
||
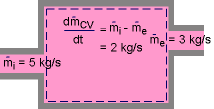 Mass Balance for Unsteady-flow Processes |
Or in rate form Also, the energy content of a control volume changes with time during an unsteady-flow process. The general energy balance can be used for the control volume as Ei - Ee = ΔECV where or in rate form where Noting that the energy can be transferred by heat, work, and mass only, the energy balance can be rewritten as where |
|
| Unform-flow Processes |
||
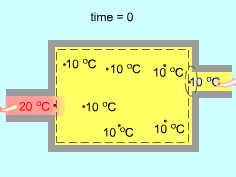 Uniform-flow Processes |
Uniform-flow processes are special cases of unsteady-flow processes. During a unform-flow process, the state of the control volume changes with time, but it does so uniformly. That is,
With these identifications, the mass and energy balances for uniform-flow processes become where |
|
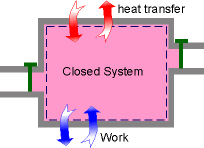 System Reduces to a Closed System when all the Inlets and Exits are Closed |
When no mass is entering or leaving the control volume, and the kinetic and potential energy changes associated with the control volume are negligible, the energy equation can be reduced to the first law relation for closed system. m2 = m1= m |
|