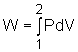| Ch 1. Basics | Multimedia Engineering Thermodynamics | ||||||
| System |
Temperature & Pressure |
Heat and Work |
Energy | ||||
| Heat and Work | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
| |
||
| Path Function and Point Function |
||
|
|
Path function and Point function are introduced to identify the variables of thermodynamics.
|
|
| Heat |
||
 Heat Transfer Direction |
Heat is energy transferred from one system to another solely by reason of a temperature difference between the systems. Heat exists only as it crosses the boundary of a system and the direction of heat transfer is from higher temperature to lower temperature. For thermodynamics sign convention, heat transferred to a system is positive; Heat transferred from a system is negative. The heat needed to raise a object's temperature from T1 to T2 is: Q = cp m (T2 - T1) where Unit of heat is the amount of heat required to cause a unit rise in temperature of a unit mass of water at atmospheric pressure.
J is the unit for heat in the S.I. unit system. The relation between Cal and J is 1 Cal = 4.184 J Notation used in this book for heat transfer:
|
|
| Modes of Heat Transfer |
||
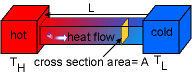 Conduction | Conduction: Heat transferred between two bodies in direct contact. Fourier's law: If a bar of length L was put between a hot object TH and a cold object TL , the heat transfer rate is: where |
|
 Convection |
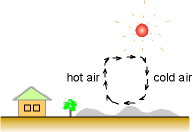
Convection: Heat transfer between a solid surface and an adjacent gas or liquid. It is the combination of conduction and flow motion. Heat transferred from a solid surface to a liquid adjacent is conduction. And then heat is brought away by the flow motion. Newton's law of cooling:
The atmospheric air motion is a case of convection. In winter, heat conducted from deep ground to the surface by conduction. The motion of air brings the heat from the ground surface to the high air. |
|
 Radiation |
Radiation: The energy emitted by matter in the form of electromagnetic waves as a result of the changes in the electronic configurations of the atoms or molecules. Stefan - Boltzmann law: where Solar energy applications mainly use radiation
energy
from the Sun. |
|
 Double Pane Window |
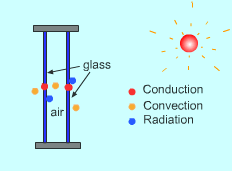
The three modes of heat transfer always exist simultaneously. For example, the heat transfer associated with double pane windows are:
Please view heat transfer books for details of modes of heat transfer. |
|
| |
Work
|
|
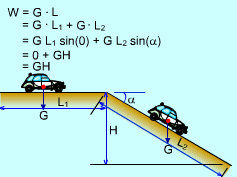 Definition of Work |
Work is the energy transfer associated with a force acting through a distance. Like heat, Work is an energy interaction between a system and its surroundings and associated with a process. In thermodynamics sign convection, work transferred out of a system is positive with respect to that system. Work transferred in is negative. Units of work is the same as the units of heat. Notation:
|
|
| Expansion and Compression Work |
||
|
|
A system without electrical, magnetic, gravitational motion and surface tension effects is called a simple compressible system. Only two properties are needed to determine a state of a simple compressible system. Considering the gas enclosed in a piston-cylinder device
with a cross-sectional area of the piston A. Initial State:
Finial State:
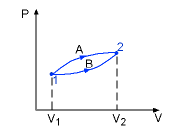
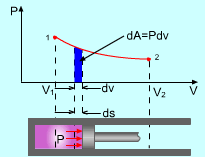
Then a work between initial and final states is:
Pressure P, Volume V. Let the piston moving ds in a quasi-equilibrium
manner. The differential work done during this process is: The total work done during the whole process (from state
(P1,V1) to state (P2,V2))
is: |
|