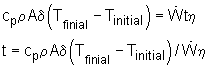| Ch 1. Basics | Multimedia Engineering Thermodynamics | ||||||
| System |
Temperature & Pressure |
Heat and Work |
Energy | ||||
| Heat and Work | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
| |
|
A certain time will be needed to warm up the iron to a certain temperature before ironing. The energy generated by the wires partly lost to the ambient by heat transfer. For a given ironing temperature, the warm up time and the quantity of heat loss need to be determined. |
|
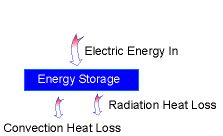 Energy Balance Diagram |
Energy balance of this warming up process: Ein - Eout = Estorage During this process, the temperature of the base becomes higher
than the ambient air temperature. So convection and radiation heat transfer
exist. Eout= Econvection + Eradiation 1. If all the energy transferred to the base is stored to increase the temperature of the base, the time will be shortest. In another word, assuming no heat loss to the ambient by convection and radiation. Ein - Eout = Estorage Estorage = Ein - Eout The heat generated by the wires is: Energy stored in the base is: Estorage = cpm (Tfinal - Tinitial) m = ρAδ Plug the numbers, the solution for time t is: t = 342 s 2. Consider the convection and radiation heat loss from the iron after
the temperature reaches 140 oC. Because temperature keeps at
140o C,
no energy is stored. 98.8/100 = 0.98 = 98.8% |