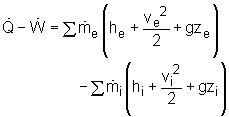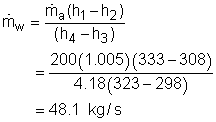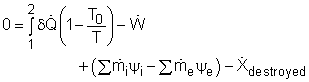| Ch 7. Exergy Analysis | Multimedia Engineering Thermodynamics | ||||||
| Exergy |
Exergy Change |
Exergy Balance(1) |
Exergy Balance(2) |
||||
| Exergy Balance (2) | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||
|
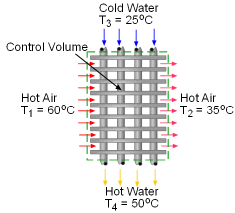
A heat recovery system is designed to heat cold water using the exhausted hot air from a subway station. Mass flow rate of the cold water, exergy destruction, and the second-law efficiency of the heat recovery system needs to be determined. Assumptions:
|
||
|
|
Consider the heat recovery system as a control volume and denote the hot air inlet as 1, hot air exit as 2, cold water inlet as 3, and hot water exit as 4, shown on the left. (1) Determine the mass flow rate of the cold water The energy balance for a control volume is: According to the assumptions, the energy balance can be simplified to Water is modeled as an incompressible substance. Thus, its enthalpy equals h4 = cwT4 and h3 = cwT3 Exhausted air is modeled as an ideal gas, its enthalpy equals h2 = cPaT2 and h1 = cPaT1 where T1,T2, T3 , T4 and
T1 = 60oC = 333 K Substituting all the data to the energy balance gives the mass flow rate of cold water. (2) Determine the exergy destruction of the heat recovery system The exergy balance of control volume undergoing a steady-flow process is
|
|
Since the heat recovery system is an adiabatic heat exchanger, it becomes where ψ is the flow exergy. it is expressed as ψ = h - T0s Therefore, the exergy destruction can be rewritten as
Substitute all the data given to the above equation gives
(3) Determine the second-law efficiency of the heat recovery system The definition of the second-law efficiency of a heat exchanger is 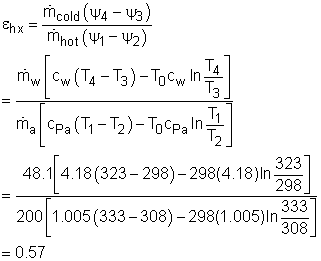 |
||