| Ch 7. Exergy Analysis | Multimedia Engineering Thermodynamics | ||||||
| Exergy |
Exergy Change |
Exergy Balance(1) |
Exergy Balance(2) |
||||
| Exergy Balance (2) | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| Search |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| Author(s): |
| Meirong Huang |
| Kurt Gramoll |
| ©Kurt Gramoll |
|
|
||
| Introduction |
||
|
|
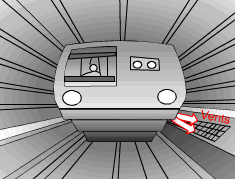
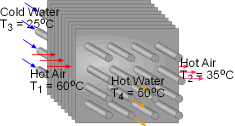
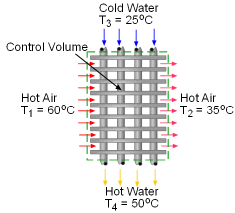
When a train in a subway approaches the station, it releases heat to its surrounding air when it brakes to stop. The heated air is exhausted through the vents under the station island. An ideal to reuse this waste heat is to design a waste heat recovery system. In this system, cold water is heated by the exhausted air. To evaluate the recovery system, exergy analysis of this system should be done. What is known:
|
|
|
|
Question |
|
|
||
| Approach |
||
|
||