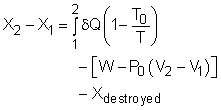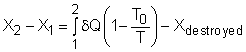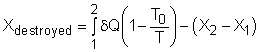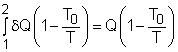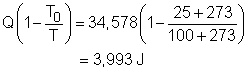| Ch 7. Exergy Analysis | Multimedia Engineering Thermodynamics | ||||||
| Exergy |
Exergy Change |
Exergy Balance(1) |
Exergy Balance(2) |
||||
| Exergy Balance (1) | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||
|
Exergy destroyed by boiling an egg from 10oC to 100oC in 100oC water needs to be determined. Assumptions:
|
||
|
|
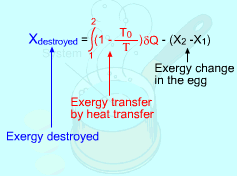
Take the egg as a system. It is a closed system since no mass flows in or out of the egg. The exergy balance for a closed system is: where 1 denotes the initial state and 2 denotes the final state. The assumption states that no work interaction is involved, and the volume of the egg does not change during the cooking process since eggs are incompressible. Thus, the exergy balance can be simplified as Rearranging the above equation gives the expression of the exergy destruction. That is, The first term on the right hand in the above equation represents the exergy transfer by heat transfer. The egg is cooked from 10oC to 100oC in 100oC water. Assuming the boundary of the egg remains at 100oC during the whole process then this term can be simplified to where T0 is the ambient temperature and is given as 25oC. T is the temperature at the boundary of the egg where heat transfer occurs. Hence,T is equal to 100oC. Q is the heat transferred to the egg from the water. Q can be determined by the energy balance of the egg. The egg is approximated as an incompressible substance and no work interaction is involved. With constant-specific-heat assumption, the energy balance is Q = mcave (T2 - T1) The mass of the egg is m = Vρ = 4/3(3.14)(0.03)3(1,000) = 0.113 kg The average specific heat is given as 3.4 kJ/(kg-K). Substituting all the data into the expression of energy balance yields
Substituting Q, T0 and T to the expression of exergy transfer by heat transfer gives, The second term on the right hand side of the exergy destruction is the exergy change in the egg during the cooking process. The exergy change of a closed system without kinetic and potential energy changes is: X2 - X1 = (U2 - U1) + P0(V2 - V1) - T0(S2 - S1) |
|
Since the egg is incompressible, the second term in the right hand of the above equation equals 0. Also, the constant-specific-heat assumption is valid. The exergy change in the egg can be determined from X2 - X1 = mCave(T2 - T1) - T0mCaveln(T2/T1) T1 is the initial temperature and T2 is the final temperature of the egg. They are given as T1 = 10oC
= 283 K Substituting all the data into the expression of exergy change in the egg yields X2 - X1 = 0.113(3,400)(373 - 283) - After calculating the exergy transfer by heat and the exergy change in the egg, the exergy destruction can be determined. Xdestroyed = 6,953.0 - 2,960.2 = 3,992.8 J Note: Another method to determine the exergy destruction is from its definition. Xdestroyed = T0Sgen The entropy generation is 13.4 J/K. (See previous section to view how to determine the entropy generation). Thus, the exergy destruction is Xdestroyed = T0Sgen = (25 + 273)(13.4) = 3,993.2 J which is almost the same as the one determined from the exergy balance. |
||