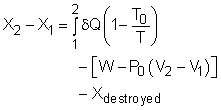| Ch 7. Exergy Analysis | Multimedia Engineering Thermodynamics | ||||||
| Exergy |
Exergy Change |
Exergy Balance(1) |
Exergy Balance(2) |
||||
| Exergy Balance (1) | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||
| Introduction |
||
|
|
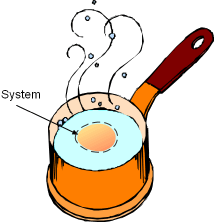
Pat is taking a thermodynamics course this semester and he tries to connect his daily life with what he learned in class. Recently, he has calculated how much entropy is generated when boiling eggs. Now, he wanders how much exergy is destroyed when the eggs are boiled. What is known:
|
|
|
|
Question |
|
How much exergy is destroyed by boiling one egg to 100oC? |
||
| Approach |
||
|
||