| Ch 3. First Law of Thermodynamics | Multimedia Engineering Thermodynamics | ||||||
|
Conservation of Mass |
Conservation of Energy |
Solids and Liquids |
Ideal Gas | ||||
| Energy Analysis of Ideal Gas | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||
|
|
Ann turns on her fan trying to cool her room while she is gone. The room temperature when she comes back in the evening needs to be determined. Assumptions:
|
|
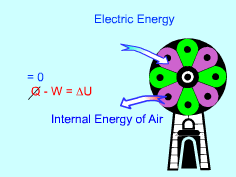 Simplification of the Energy Balance Equation |
The system is a closed system since all the doors and windows are tightly closed. It is a stationary system by disregarding the changes of the velocity and elevation of air during the cooling process. The energy balance for this system is: Q - W = U2 - U1 Also, the assumption indicates that the heat transfer through the walls and windows is disregarded, hence no heat transfer to or from this system. Q = 0 The electric energy is transferred into the system by the fan, hence this work is negative. W = - For an ideal gas, the internal energy is a function of the temperature. If the specific heat at the average room temperature is used, the difference of internal energy is: U2 - U1 = cv,av(T2 - T1) The energy equation for the air in the room becomes: T2 = Ann leaves at 8:00 in the morning and returns at 6:00 in the evening. Hence the total time is 10 hours. The average value of specific heat is used to calculate the temperature. A room temperature is assume first. Then iteration is used till a reliable result obtained. Assume the room temperature is 55 oC when she is back in the evening. Tav = (15 + 55)/2.0 = 35 oC At 1 atm and 35 oC, the density of the air is ρ = 1.166 kg/m3. The mass of the air in the room is: m = (4)(6)(6)(1.166) = 167.9 kg |
|
|
Specific Heats of Some Common Ideal Gases |
From table, at 35 oC, the constant volume specific heat is With all the data known, T2 = (150)(10)(3600)/((718)(167.9) + 15 = 59.8 oC Tav = (15 + 59.8)/2.0 = 37.4 oC The calculated average temperature is pretty close to 35 oC (the assumed temperature), hence no iteration is needed. As a result, it looks like Ann's dream of a cooler room in the evening is impossible after all. |
|