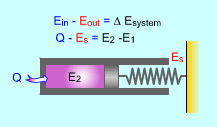THERMODYNAMICS - THEORY
|
| |
|
The First Law of Thermodynamics
|
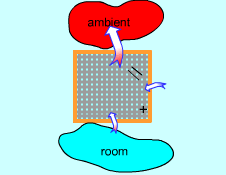
Conservation of Energy for an
Air Conditioner |
|
The first law of thermodynamics, also known as the conservation of energy principle, states:
Energy can be neither created nor destroyed; it can only change forms. |
The first law is based on experimental observations. It can not be proved
mathematically, but no known process in nature violates the first
law.
|
| |
|
| |
|
Energy Balance
|
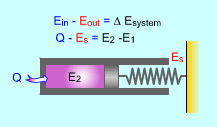
Energy Balanced for a
Piston-cylinder Spring Device |
|
During a process, the first law can be expressed as:
the net change in the total energy during a process is equal to the difference
between the total energy entering and leaving the
system during that process. In an equation format, the energy balance
for a system is:
(Total energy entering the system)
-
(Total energy leaving the system)
=
(Net change in the total energy of the system)
or,
Ein- Eout = ΔEsystem
The rate form of the energy balance of a system is:
( Rate at which energy entering the system)
-
(Rate at which energy leaving the system)
=
(time rate of change in the total energy of the system)
or,
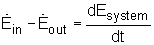 |
| |
|
|
| |
|
Mechanisms of Energy
Transfer Ein and Eout
|
|
|
Energy is transferred to or from a system by three forms: heat, work
and mass flow. For a closed system, the only two forms are heat and work
since no mass crosses its boundaries.
|
| |
|
|
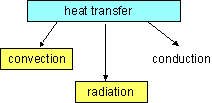
Modes of Heat Transfer
|
|
1. Heat transfer (Q)
Heat transfer is the energy interaction caused by a temperature difference
between a system and its surroundings. Heat transfer to a system (heat
gain) will cause
the internal energy of the system to increase, and heat transfer from a
system (heat loss) will cause the internal energy of the system to decrease.
|
| |
|
|
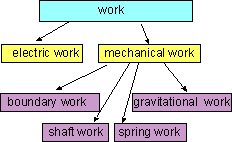
Some Forms of Work |
|
2. Work (W)
The energy interaction that is not caused by a temperature difference between
a system and its surroundings is work. Work transferred to a system will
increase the energy of the system, and work transferred from a system
will decrease
the energy of the system.
3. Mass flow
When mass enters a system, the energy of the system increases because of the
energy accompanied by mass. Also the energy of
the system decreases when mass
leaves the system. |
| |
|
|
| |
|
Heat and work have been introduced in the previous sections. Systems
that involve energy balance with mass flow are considered in the following
section.
Note that net transfer of a quantity is equal to the difference
between the amounts transferred in and out, the energy balance can
be written as:
(Qin - Qout ) + (Win - Wout)
+ (Emass,in - Emass,out) = ΔEsystem
All the quantities of the six terms in the left are positive amounts
since the direction of the energy transfer is described by the subscripts "in" and "out".
|
| |
|
|
| |
|
Energy Change of a System Δ Esystem
|
| |
|
The energy change of a system during a process is:
Energy change
=
Energy at final state
-
Energy at initial state
or,
ΔEsystem =
Esystem@final - Esystem@initial
From the discussion in the previous section, it is known that the change
in the total energy of a system during a process is the sum of the changes
in its internal, kinetic, and potential energies.
ΔE = ΔU + ΔKE + ΔPE |
| |
|
|