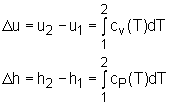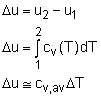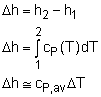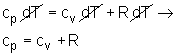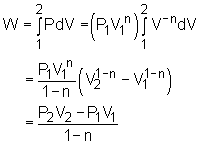| Ch 3. First Law of Thermodynamics | Multimedia Engineering Thermodynamics | ||||||
|
Conservation of Mass |
Conservation of Energy |
Solids and Liquids |
Ideal Gas | ||||
| Energy Analysis of Ideal Gas | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
| |
||||||||||||||||||||||
| Internal Energy, Enthalpy, and Specific Heats of Ideal Gas |
||||||||||||||||||||||
|
|
The ideal gas is defined as a gas which obeys the following equation of state: Pv = RT The internal energy of an ideal gas is a function of temperature only. That is, u = u(T) Using the definition of enthalpy and the equation of state of ideal gas to yield, h = u + P v = u + RT Since R is a constant and u = u(T), it follows that the enthalpy of an ideal gas is also a function of temperature only. h = h(T) Since u and h depend only on the temperature for an ideal gas, the constant volume and constant pressure specific heats cv and cp also depend on the temperature only. cv = cv (T) cP = cP (T) For an ideal gas, the definitions of cv and cp are given as follows: and they can be rewritten as cv = du/dT cP = dh/dT During a process from state 1 to state 2, the changes of internal energy and enthalpy are: |
|||||||||||||||||||||
Specific Heats of Common Ideal Gases |
There are three ways to determine the changes of internal energy and enthalpy for ideal gas.
|
|||||||||||||||||||||
| Specific Heat Relations of Ideal Gas
|
||||||||||||||||||||||
Combing the definition of enthalpy and the equation of state for ideal gas to yield h = u + pv = u + RT Differentiating the above relation to give dh = du + RdT Replacing dh by cPdT and du by cvdT, This is a special relationship between cv and cP for an ideal gas. Also, the ratio of cP and cv is called the specific heat ratio, k = cP/cv The specific heat ratio is also a temperature dependent property. For air at T = 300 K, cP =
1.005 kJ/(kg-K) |
||||||||||||||||||||||
| The Polytropic Process |
||||||||||||||||||||||
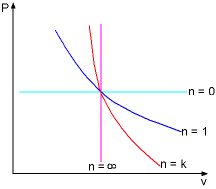 Special Processes of Ideal Gas on a P-v Diagram |
Many processes which occur in practice can be described by an equation of the form Pvn = constant where For a process from state 1 to state 2, the relation is: P1v1 n = P2v2n By using the equation of state of ideal gas, the relations between P, v, and T are: T2/T1=(P2/P1)(n-1)/n P2/P1=(v1/v2)n For some specific values of n, the process becomes isobaric, isothermal, isometric, and adiabatic, and they are summarized as follows:
|
|||||||||||||||||||||
| Energy Analysis for Ideal Gas in a Closed System at
Different Processes |
||||||||||||||||||||||
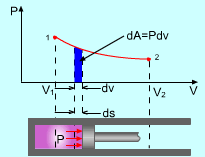 Boundary Work |
The energy balance for a stationary, closed system is: Q - W = ΔU For ideal gas, the internal energy can be determined by ΔU = U2 - U1 = cv(T2 - T1) where If only boundary work is considered, then the work W can be determined by For a polytropic process, the work W is: For special processes such as the isobaric, isothermal,
isometric, and adiabatic processes for ideal gas, using the average specific
heats, the heat, work, and internal energy are given in the following table: |
|||||||||||||||||||||
|
||||||||||||||||||||||