| Ch 2. Pure Substances | Multimedia Engineering Thermodynamics | ||||||
| Phase |
Property Diagrams |
Property Tables |
Ideal Gas |
||||
| Property Tables | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| Search |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| Author(s): |
| Meirong Huang |
| Kurt Gramoll |
| ©Kurt Gramoll |
|
|
||
|
|
500 kg of water is in a 10 m3 boiler under 5 Mpa. The
temperature, enthalpy, and the mass of each phase of water are needed
to monitor the vaporization process. |
|
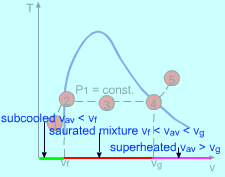 Steps to Determine the States |
(1) Determining the state of water The specific volume of the water is vav = V/m = 10/500 = 0.02 m3/kg From the saturated water table, find the specific volume for saturated liquid vapor at 5 Mpa. vf = 0.001286 m3/kg vg = 0.03944 m3/kg vf = 0.001286 < vav = 0.02< vg = 0.03944 m3/kg Since vf < vav< vg, the water is in saturated mixture state. |
|
| Saturated Water Pressure Table | (2) Temperature Since the water is a saturated mixture, the temperature is the saturation temperature. From the saturated water table, the saturation temperature at 5 Mpa is 263.99oC. (3) Quality x The quality of the saturated mixture is given by x = (vav - vf)/vfg vfg = vg - vf = 0.038154 m3/kg x = (0.02 - 0.001286)/0.038154 = 49% (4) Enthalpy Once again, from the saturated water table, hf and hg can be found at P = 5 Mpa. hf = 1,154.23 kJ/kg hg = 2,794.3 kJ/kg hfg = hg - hf = 1,640.1 kJ/kg hav = hf + xhfg = 1,154.23 + (49%)(1,640.1) = 1,958 kJ/kg (5) Mass of each phase The definition of quality is given by x = mg/mtotal The mass for each phase can be calculated as mg = x mtotal = ( 49%)(500) = 245 kg ml = mtotal - mg = 500 - 245 = 255 kg |
|