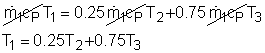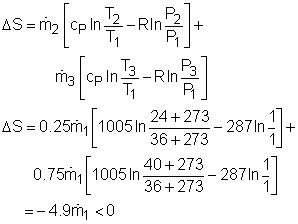| Ch 6. Entropy | Multimedia Engineering Thermodynamics | ||||||
| Entropy | Tds Relations |
Entropy Change |
Isentropic Process |
Isentropic Efficiency |
Entropy Balance (1) |
Entropy Balance (2) |
Reversible Work |
| Entropy Change | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||
|
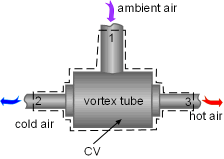
A salesperson states that this spot cooler can work at the atmospheric pressure without any energy consumption. Evaluate his claim. Assumptions:
|
||
|
Take the spot cooler as a control volume shown on the left. If the spot cooler satisfies both the first and the second law of thermodynamics, it will work. To verify if it satisfies the first law, check the energy balance of the system. To verify if it satisfies the second law, check the entropy change of the entire process. With all the assumptions, the mass and energy balance of the control volume are: The mass balance is satisfied since The constant-specific-heat assumption is also used. So with constant specific heats, the energy balance becomes Substituting the temperatures into both sides of the above equation yields right side = 0.25(24 + 273) +0.75(40 + 273) = 309 The result above shows the energy balance is satisfied. To check if the spot cooler satisfies the second law, calculate the entropy change of the entire process. If the entropy change is not negative, the process can happen. With constant specific heats, the entropy change equals The result shows that the spot cooler cannot work at atmospheric pressure. The air at the inlet needs to be compressed to a higher pressure to make the cooler work, which requires energy input. |
|