| Ch 5. Second Law of Thermodynamics | Multimedia Engineering Thermodynamics | ||||||
| Heat Engine |
The Second Law |
Carnot Cycle |
Carnot Heat Engine |
Carnot Refrigerator |
|||
| The Carnot Cycle | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
| |
||
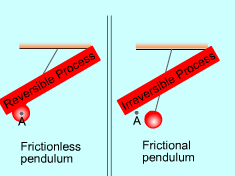
| Reversible and Irreversible Process
|
A process is reversible if, after it has been carried out, it is possible to restore both the system and its entire surroundings to exactly the same states they were in before the process. If the system and its surroundings cannot return to their initial states at the end of the reversed process, this process is an irreversible process. A system can be restored to its initial state following a process, regardless if the process is reversible or not. If the surroundings can also be restored to its initial state, the process is reversible. Otherwise, the process is irreversible. Reversible process does not occur in nature. It is the idealization of actual process and serves as an idealized model to which actual process can be compared. The factors that cause a process to be irreversible are called irreversibilities. They include:
The process is irreversible if any of these effects present. |
|
| Internally and Externally Reversible Processes
|
||
When a process is carried out, irreversibilities can be found within the system as well as in the system's surroundings. A process is called internally reversible if the system can be restored through exactly the same equilibrium states which the system goes through. No irreversibilities occur within the boundaries of the system as it goes through the process. If no irreversibilities occur outside the system boundaries during the process, the process is called externally reversible. A process is called totally reversible, or reversible, if it is both
internally and externally reversible. |
||
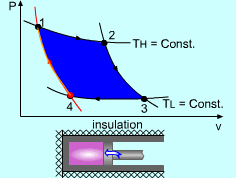
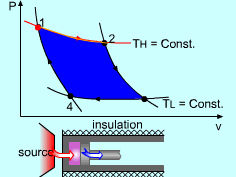
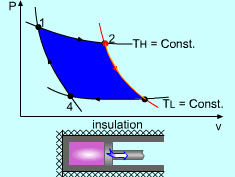
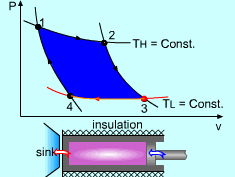
| The Carnot Cycle
|
|
Heat engine operates on a cycle. The efficiency of heat engine depends on how the individual processes are executed. The most efficient cycles are reversible cycles, that is, the processes that make up the cycle are all reversible processes. Reversible cycles cannot be achieved in practice. However, they provide the upper limits on the performance of real cycles. Carnot cycle is one of the best-known reversible cycles. The Carnot cycle is composed of four reversible processes. Consider an adiabaticpiston-cylinder device that contains gas. The four reversible processes that make up the Carnot cycle are as follows:
|
| The Carnot Principles
|
||
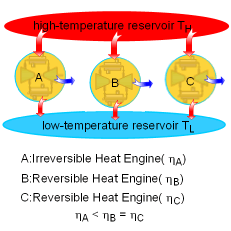 Carnot Principles |
If the processes that make up the cycle of the heat engine are all reversible processes, the heat engine is a reversible heat engine. Otherwise, it is an irreversible heat engine. In practice, all heat engines are irreversible since no reversible process exists in nature. The Carnot Principles are conclusions of the second law of thermodynamics. They are expressed as follows:
|
|
 <
<
 <
<