| Ch 4. Energy Analysis | Multimedia Engineering Thermodynamics | ||||||
|
Steady-flow Process |
Steady-flow Devices (1) |
Steady-flow Devices (2) |
Steady-flow Devices (3) |
Unsteady-flow Process |
|||
| Unsteady-flow Process | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||
| Introduction |
||
|
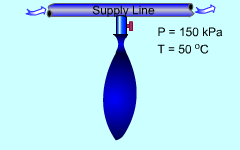
Minnie volunteers to help the new semester welcoming party. Her job is to fill the balloons with helium from a supply line. The balloons have some limitations that she needs to consider when she fills them. What is known:
|
||
| Questions |
||
|
||
| Approach |
||
|
|
|