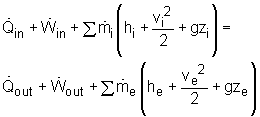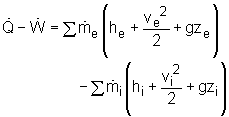| Ch 4. Energy Analysis | Multimedia Engineering Thermodynamics | ||||||
|
Steady-flow Process |
Steady-flow Devices (1) |
Steady-flow Devices (2) |
Steady-flow Devices (3) |
Unsteady-flow Process |
|||
| Steady Flow Process | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
| |
||
| A control volume may involve one or more forms of work at the same time. If the boundary of the control volume is stationary, the moving boundary work is zero, and the work terms involved are shaft work and electric work. Another work form with the fluid is flow work. | ||
| Flow Work
(Flow Energy)
|
||
|
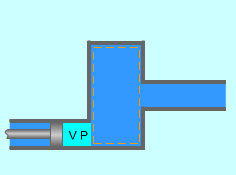
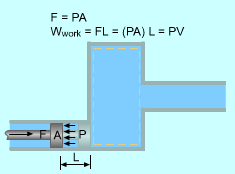 Flow Work with Imaginary Piston |
Work is needed to push the fluid into or out of the boundaries of a control volume if mass flow is involved. This work is called the flow work (flow energy). Flow work is necessary for maintaining a continuous flow through a control volume. Consider a fluid element of volume V, pressure P, and cross-sectional area A as shown left. The flow immediately upstream will force this fluid element to enter the control volume, and it can be regarded as an imaginary piston. The force applied on the fluid element by the imaginary piston is: F = PA The work done due to pushing the entire fluid element across the boundary into the control volume is Wflow = FL = PAL = PV For unit mass, wflow = Pv The work done due to pushing the fluid element out of the control volume
is the same as the work needed to push the fluid element into the control
volume. |
|
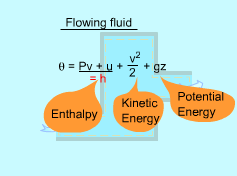
| Total Energy of a Flowing Fluid
|
|
The total energy of a simple compressible system consists of three parts: internal, kinetic, and potential energy. E = U + KE + PE For unit mass, e = u + ke + pe = u + v2/2 + gz where The fluid entering or leaving a control volume possess an additional energy, the flow work (Pv). Hence, the total energy of a flowing fluid becomes θ = Pv + u + v2/2 + gz where The definition of enthalpy gives h = Pv + u Replacing Pv + u by h yields θ = h + v2/2 + gz By using the enthalpy instead of internal energy, flow work is not a concern. |
| The Steady-flow Process
| ||
|
|
Steady flow process is a process where: the fluid properties can change from point to point in the control volume but remains the same at any fixed point during the whole process. A steady-flow process is characterized by the following:
|
|
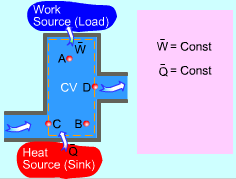
| Mass and Energy Balance for Steady-flow Process
| ||
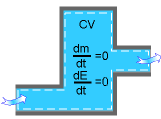
The conservation of mass principle, which has been previously introduced, in rate format, is: During a steady-flow process, the total amount of mass contained within a control volume does not change with time. That is, dmsystem/dt = 0 Hence the conservation of mass principle gives the total amount of mass entering a control volume equal to the total amount of mass leaving it. In an equation format, it is |
||
 Mass and Energy balance for Steady-flow Process |
(Total mass entering the control volume per unit time) or, where Also, the energy balance for a process, which has been previously introduced, in rate format, is:
For a steady-flow process, the total energy content of a control volume remains constant. That is, dEsystem/dt = 0 Thus, the amount of energy entering a control volume in all forms (heat, work, mass transfer) must be equal to the amount of energy leaving it for a steady-flow process. In an equation format, it is (Rate of net energy transfer in by heat, work and mass) or, For a general steady-flow process, the energy balance can be written as If the sign introduced previously for heat and work is used, the energy balance for a general steady-flow process can be rewritten as: |
|