| Ch 1. Basics | Multimedia Engineering Thermodynamics | ||||||
| System |
Temperature & Pressure |
Heat and Work |
Energy | ||||
| System | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
| THERMODYNAMICS - THEORY |
||
| Systems |
||
 System and Surroundings |
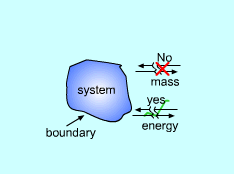
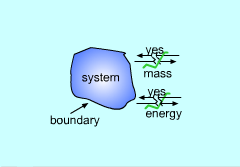
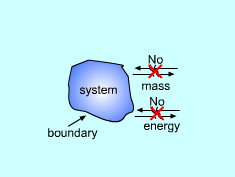
All thermodynamic systems contain three basic elements:
|
|
|
Systems can be classified as being closed, open, or isolated.
|
|
| Property, Equilibrium and State |
||
 Two States of a System |
A property is any measurable characteristic of a system. The common properties include:
Properties can be intensive or extensive. Intensive properties are those whose values are independent of the mass possessed by the system, such as pressure, temperature, and velocity. Extensive properties are those whose values are dependent of the mass possessed by the system, such as volume, enthalpy, and entropy (enthalpy and entropy will be introduced in following sections). Extensive properties are denoted by uppercase letters, such as volume (V), enthalpy (H) and entropy (S). Per unit mass of extensive properties are called specific properties and denoted by lowercase letters. For example, specific volume v = V/m, specific enthalpy h = H/m and specific entropy s = S/m (enthalpy and entropy will be introduced in following sections). Note that work and heat are not properties. They are dependent of the process from one state to another state. When the properties of a system are assumed constant from point to point and there is no change over time, the system is in a thermodynamic equilibrium. The state of a system is its condition as described by giving values to its properties at a particular instant. For example, gas is in a tank. At state 1, its mass is 2 kg, temperature is 20oC, and volume is 1.5 m3. At state 2, its mass is 2 kg, temperature is 25oC, and volume is 2.5 m3. A system is said to be at steady state if none of its properties changes with time. |
|
| Process, Path and Cycle |
||
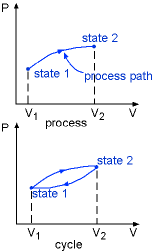 Process and Cycle |
The changes that a system undergoes from one equilibrium state to another is called a process. The series of states through which a system passes during a process is called path. In thermodynamics the concept of quasi-equilibrium processes is used. It is a sufficiently slow process that allows the system to adjust itself internally so that its properties in one part of the system do not change any faster than those at other parts. When a system in a given initial state experiences a series of quasi-equilibrium processes and returns to the initial state, the system undergoes a cycle. For example, the piston of car engine undergoes Intake stroke, Compression stroke, Combustion stroke, Exhaust stroke and goes back to Intake again. It is a cycle. |
|