| Ch 1. Basics | Multimedia Engineering Fluids | ||||||
|
Mass Density |
Ideal Gas Law |
Viscosity |
Surface Tension |
Vapor Pressure |
|||
| Mass Density and Specific Weight | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Fluid Statics |
| 3. Kinematics |
| 4. Laws (Integral) |
| 5. Laws (Diff.) |
| 6. Modeling/Similitude |
| 7. Inviscid |
| 8. Viscous |
| 9. External Flow |
| 10. Open-Channel |
| Appendix |
| Basic Math |
| Units |
| Basic Fluid Eqs |
| Water/Air Tables |
| Sections |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||||||||||||||
The raw data collected are given as follows:
The mass of the liquid is determined by subtracting the mass of
the empty container from the mass of the container with liquid. The density
of the liquid is then determined by dividing the mass by its volume. For Liquid B: |
||||||||||||||
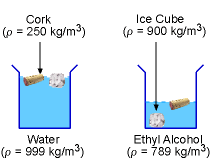 Ice Cube and Cork in Water and Ethyl Alcohol |
By comparing the computed densities with the density values found in standard handbooks, it is possible that liquid A is water while liquid B is ethyl alcohol (note that other liquids or mixtures may have the same densities). The density of the ice cube (900 kg/m3) is less than the water's density, but it is greater than the ethyl alcohol's density. Hence, the ice cube will float on water while it will sink in ethyl alcohol. On the other hand, a cork of density 250 kg/m3 is less dense than the water and the ethyl alcohol. Hence, it will float on both the liquids. |
|||||||||||||