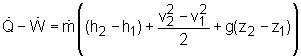| Ch 4. Energy Analysis | Multimedia Engineering Thermodynamics | ||||||
|
Steady-flow Process |
Steady-flow Devices (1) |
Steady-flow Devices (2) |
Steady-flow Devices (3) |
Unsteady-flow Process |
|||
| Steady-flow Devices (2) | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| Search |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| Author(s): |
| Meirong Huang |
| Kurt Gramoll |
| ©Kurt Gramoll |
|
|
||
|
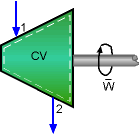
A hydraulic turbine is used to produce power using for the apartments' lighting. The power generated needs to be determined. Assumptions:
|
||
|
|
The energy balance for one-inlet-one-exit system is: Applying the basic assumptions for turbines, the energy balance can be rewritten as: Water enters the turbine at 300 K and 750 kPa. At 300 K and 750 kPa, water is compressed liquid. The enthalpy of compressed liquid can be approximated by h = hf@T + vf (P - Psat.) where From the water table, the saturated properties of water at 300 K are: hf@300 k = 104.89 kJ/kg Hence, enthalpy at 300 K and 750 kPa is: h1 = 104.89 + 0.001003(750 - 3.169) At 300 K and 170 kPa, water is compressed liquid also. Using the same approximation, the enthalpy of water at 300 K and 170 kPa is h2 = 104.89 + 0.001003(170 - 3.169) Substituting these enthalpies and the given mass flow rate to the energy balance equation yields - The power is generated by the system, so it is positive. The power needs for lighting an apartment is 500 W, which is smaller than the power generated by the turbine. 500 W < 1.6 kW = 1,600 W Hence, the result shows the turbine can be used to generate power for the lighting system. |
|