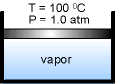| Ch 2. Pure Substances | Multimedia Engineering Thermodynamics | ||||||
| Phase |
Property Diagrams |
Property Tables |
Ideal Gas |
||||
| Phase and Phase Change | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| Search |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| Author(s): |
| Meirong Huang |
| Kurt Gramoll |
| ©Kurt Gramoll |
| |
||
| Pure Substance |
||
 Pure Substance: Ice and Water |
A substance that has a fixed chemical composition throughout is called a pure substance. Examples of pure substances:
Examples of non-pure substances:
|
|
| Solid, Liquid, and Gas |
||
Substances exist in different phases. A phase is identified as having a distinct molecular arrangement that is homogeneous throughout and separated from other phases by easily identifiable boundary surface. The three principal phases are solid, liquid, and gas. Solid: The large attractive forces of molecules on each other keep the molecules at fixed position. Ice is the solid phase of water. Liquid: Chunks of molecules float about each other. The molecules maintain an orderly structure within each chunk and remain their original positions with respect to one another. Water in room temperature and 1 atm pressure is in liquid phase. Gas: Molecules are far apart from each other and move about at random.
Air is in gaseous phase in room temperature and 1 atm pressure. |
||
| Latent Heat |
||
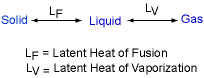 Latent Heat |
When a material changes from a solid to liquid, or from a liquid to a gas, an amount of energy is involved in the change of phase. This energy must be supplied or removed from the system to cause the molecular rearrangement. This energy is called the latent heat. Latent heat relative to melting a solid is called the latent heat of
fusion (LF).
Latent heat relative to vaporizing a liquid
is called the latent heat of vaporization (LV). For example,
when ice at 1 atm is melted to water
at 0 oC, the latent heat of fusion is 333 kJ/kg.The same
quantity of heat will be removed for freezing a pound of
water to
ice.
Liquid water
boils into vapor at 100 oC, the latent heat of vaporization
is 2257 kJ/kg. Also the same quantity of heat will be removed when condensing
a pound
of water
vapor to liquid
water at this condition. |
|
| Phase-change Processes |
||
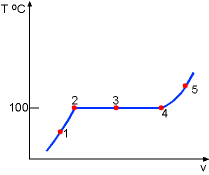
| Consider a piston-cylinder device containing liquid water at 20 oC and 1 atm. At this state, the water is in liquid phase and is called compressed liquid or subcooled liquid. |
||
| While keeping the pressure constant which is 1.0 atm, add heat to the piston-cylinder device till the temperature reaches 100 oC. If additional heat is added to the water, vapor will appear. The liquid water at this state is called saturated liquid. | ||
Continuing to add heat to the piston-cylinder device, the liquid will vaporize. The piston-cylinder contains both liquid water and vapor. It is called saturated liquid-vapor mixture or saturated mixture. |
||
| The temperature remains at 100 oC if the liquid and vapor coexist. The vapor is called saturated vapor just when all liquid becomes vapor. | ||
 Superheated vapor |
The temperature of the vapor will rise if more heat is added to the piston-cylinder system after it reaches the saturated vapor state. The vapor for which temperature is higher than that of saturated vapor is called superheated vapor. | |
|
The entire process can be described on a T-v diagram shown on the left.
|
|