| Ch 2. Pure Substances | Multimedia Engineering Thermodynamics | ||||||
| Phase |
Property Diagrams |
Property Tables |
Ideal Gas |
||||
| Property Tables | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
| |
||
|
For most substances, thermodynamic properties are presented in the form of tables because they are too complex to be expressed by simple equations. In thermo-systems, many working fluids can be used. Water is one of the most common working fluids involved. It is the only liquid presented in this section. The thermodynamic properties that are commonly used are:
|
|
| Saturated Liquid and Saturated Vapor |
||
|
|
At a given pressure, the temperature at which a pure substance changes phase is called the saturation temperature. At 1 atm, the saturation temperature of water is 100 oC. At a given temperature, the pressure at which a pure substance changes phase is called the saturation pressure. At 100 oC, the saturation pressure of water is 1 atm. The saturation temperature and saturation pressure depends on each other. There are two types of tables for saturated water. Both tables give the same information. The only difference is that the properties are listed either as a function of temperature or pressure. The properties listed in the tables include
|
|
| Saturated Mixture |
||
During the vaporization process of water, the substance is a mixture of saturated liquid and saturated vapor. Quality (x) is defined to describe the fraction of saturated vapor in the mixture. x = mvapor/mtotal
Quality has a value between 0 and 1. x equals 1 for saturated vapor and x equals 0 for saturated liquid according to its definition. Saturated mixture is a two-phase system. For convenience, it can be treated as a homogenous mixture and the properties of this mixture is simply the average properties of the saturated liquid and saturated vapor. For example, the specific volume of the mixture can be determined by |
||
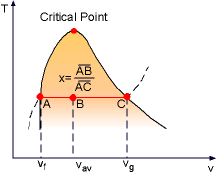 Quality is related to the horizontal distance on T-v diagram |
Vav = Vf +
Vg Rearranging the above equation to give an expression for the quality x as x = (vav - vf)/vfg Based on this equation, quality can be determined from the horizontal distance on the T-v or P-v diagram as shown in the figure. The above process can be repeated for u and h. uav = uf +
ufg x |
|
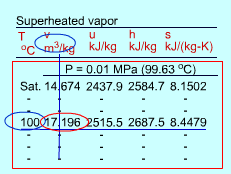
| Superheated Vapor |
||
|
|
Since the superheated vapor is a single phase substance, the temperature and pressure are independent. In the superheated vapor tables, the properties are listed as a function of both the temperature and pressure. The tabulated properties include v, u, h and s. The saturation temperature corresponding to the pressure is given in the parentheses following the pressure value. Superheated vapor can be characterized by
|
|
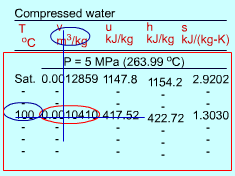
| Subcooled Liquid |
||
|
|
The format of the subcooled liquid water tables is similar to the format of the superheated vapor tables. In the absence of compressed liquid data, a common practice is to use the saturated liquid data based on the given temperature to estimate the properties of compressed liquid. For h, the error can be reduced using the following approximation Subcooled liquid can be characterized by
|
|
|
||
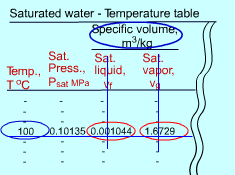 Saturated Water Table
Saturated Water Table