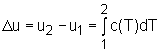| Ch 3. First Law of Thermodynamics | Multimedia Engineering Thermodynamics | ||||||
|
Conservation of Mass |
Conservation of Energy |
Solids and Liquids |
Ideal Gas | ||||
| Solids and Liquids | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
| |
||
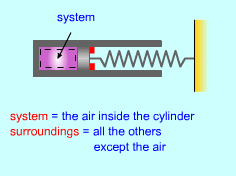
| Energy Balance for Closed Systems |
||
 Energy Balance for Closed System |
The energy balance for a system, which has been previously introduced, is: (Qin - Qout ) + (Win - Wout)
+ For a closed system, the only forms of energy that can be supplied or removed from a system are heat and work. (Qin - Qout ) + (Win - Wout) = (ΔU
+ ΔKE + ΔPE)system |
|
|
|
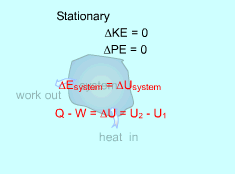
If the adopted sign convention is such that the heat entering the system is positive, and the work done by the system is positive for a process from state 1 to state 2, then the energy balance for a closed system becomes: Q - W = E2 - E1 = ΔEsystem For a stationary system, in which no velocity and elevation changes during a process, the change of the total energy of the system is due to the change of the internal energy only. That is, Q- W = U2 - U1 |
|
| Specific Heats of Solids and Liquids
|
||
The definitions of constant volume and constant pressure specific heats have been introduced previously. They are For most solids and liquids, they can be approximated as incompressible substances, hence the constant volume and constant pressure specific heats are the same. That is, cP = cv = c cv = du/dT cP =
dh/dT |
||
| Internal Energy and Enthalpy Difference of Solids
and Liquids
|
||
From the definition of specific heat, the change of internal energy becomes du = cvdT = c(T) dT For a process from state 1 to state 2, the change of internal energy is obtained by integrating the above equation from state 1 to state 2. |
||
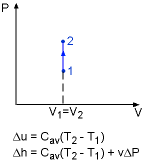 Internal Energy and Enthalpy of Solids and Liquids |
For small temperature intervals, an average specific heat (c) at the average temperature is used and treated as a constant, yielding Enthalpy is another temperature dependent variable. The definition of enthalpy is: H = U + PV It can be rewritten in terms of per unit mass as follows: h = u+ Pv Note that v is a constant, so the differential form of the above equation is: Integrating from state 1 to state 2 yields Δh = Δu + v ΔP For solids, the term vΔP is insignificant. Δh = Δu For liquids, two cases are encountered. They are:
|
|