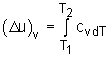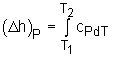| Ch 1. Basics | Multimedia Engineering Thermodynamics | ||||||
| System |
Temperature & Pressure |
Heat and Work |
Energy | ||||
| Energy, Specific Heat & Enthalpy | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| Search |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| Author(s): |
| Meirong Huang |
| Kurt Gramoll |
| ©Kurt Gramoll |
| |
||
| Energy |
||
 Chemical Energy Transfers to Kinetic Energy in Rocket |
Energy is the capacity for doing work. It may exist in a variety of forms such as thermal, mechanical, kinetic, potential, electric, magnetic, chemical, and nuclear. It may be transferred from one type of energy to another. For example,
|
|
| Forms of Energy |
||
 Kinetic Energy and Gravitational
Kinetic Energy and Gravitational Potential Energy |
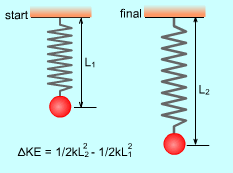
Kinetic Energy (KE): The energy that a system possesses as a result of its motion. KE = mv2/2 where If an object of mass m changes velocity from v1 to v2.
thus the change of its kinetic energy is: |
|
Potential Energy (PE): The energy that a system possesses as a result of its elevation in a gravitational field or change of configurations. Gravitational potential energy (elevation in a gravitational field): PE = mgz where Moving an object from location A to B, its gravitational potential energy change is: ΔPE = mg(ZB - ZA) |
||
|
|
Elastic potential energy (change of configurations): PE = 1/2 kx2 where |
|
 Molecules Random Movement |
Internal energy (U): The energy associated with the random, disordered motion of molecules. It is the sum of the kinetic and potential energies of all molecules.
|
|
Total Energy (E): The sum of all forms of energy exist in a system. The total energy of a system that consists of kinetic, potential, and internal energies is expressed as: E = U + KE + PE = U + mv2/2 + mgz The change in the total energy of a system is: ΔE = ΔU
+ ΔKE + ΔPE |
||
| Enthalpy (H) |
||
Enthalpy is a thermodynamics property of a substance and is defined as the sum of its internal energy and the product of its pressure and volume. H = U + PV |
||
| Specific Heat (c) |
||
| Experiment shows that the temperature rise of liquid water due to heat transfer to the water is given by Q = m c (T2 - T1) where In general, the value of specific heat c depends on the substance in the system, the change of state involved, and the particular state of the system at the time of transferring heat. Specific heat of solids and liquids is only a function of temperature but specific heat of gaseous substances is a function of temperature and process. |
||
| Specific Heat at Constant Volume (cv) |
||
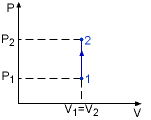 Isochoric Process |
Specific heat at constant volume is the change of specific internal energy with respect to temperature when the volume is held constant (Isochoric process). For constant volume process: |
|
| Specific Heat at Constant Pressure (cP) |
||
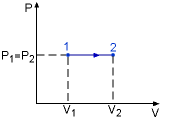 Isobaric Process |
Specific heat at constant pressure is the change of specific enthalpy with respect to temperature when the pressure is held constant (Isobaric process). For constant pressure process |
|